Why us?
Meeting
Deadlines

Guaranteed Approval
VIP
Service

Peace
of Mind
Problems We Solve
How we work
Are You a Fit?
-Who We Serve
Medical Device Manafacturers
Small/medium
Enterprise


The CiteMed Edge

The CiteMed Edge
What makes our approach unique is our blend of world class regulatory talent, leading regulatory software tools, and (almost) obsessive customer service. Here are some of the highlights:
Your Literature-Driven
Regulatory Process
Consistent and effective Literature Search/Review strengthens your entire
regulatory workflow.
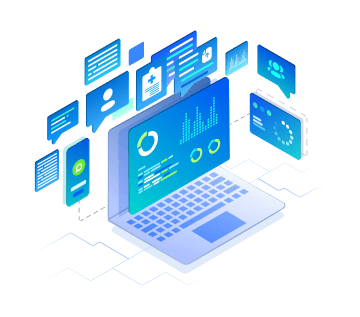
Cite Medical Literature Search
Dashboard
Our team works with our proprietary platform
to search, process, and store all scientific
literature related to your devices.

Our Trending Publications
The DIY Clinical Evaluation Report – Do You Really Need A CER Writer?
So you’ve taken a look at EU MDR transitions for some of your devices and shopped around for quotes on help writing the technical file. One of the more common [...]
Navigating Global Medical Device Regulations
Understanding the Landscape of Global Medical Device Regulations The global landscape of medical device regulations is as diverse as it is complex. Navigating through these regulations requires a deep [...]
Navigating Medical Device Safety, Regulation, and Adverse Europe
Adverse Events in Europe The landscape of medical device safety in Europe is significantly influenced by the occurrence and management of adverse events. An adverse event in this context [...]
Medical Device Regulations – Links You Should Be Aware Of
Medical device regulations are no joke. It's pretty important to get the regulations right, since even a single missed data, if significant enough, can result in years of delay [...]